Learn why implementing quality in manufacturing is crucial for product creation, risk management, and more.
Quality in manufacturing depends on effective quality control (QC), a set of procedures used to measure and test products for compliance. The main function of QC is to ensure that all goods are free of defects, meet client expectations, and adhere to industry best practices.
Products have the potential either to increase customer satisfaction or to create legal and financial complications if deficiencies are found. In the current era where consumers are increasingly conscious of product safety and quality, it is paramount that manufacturers are doing all they can to ensure products meet all quality standards. Quality goods can affect a business’s success and advance its credibility to the public. They can also lead to fewer production costs and increases in profit.
With emerging digital technologies such as AI and connected worker solutions, manufacturers can improve quality control, decrease defects, and more. AI-powered connected worker platforms allow manufacturers to standardize quality control, resulting in fewer errors, reduced defects, and streamlined quality processes that are faster and more accurate.
Explore the following content to get a better idea of why quality is crucial and ways to improve it:
- Defining quality on industrial frontlines
- Quality affects every facet of production
- How to improve quality in manufacturing
- Digitizing quality control with Augmentir
Defining quality on industrial frontlines
Quality in the manufacturing realm is all about following procedures to meet product and compliance specifications. Once the standard for quality is set, the rest is about meeting product expectations through standardized procedures.
Quality in production can be broken down into three factors: design, quality control, and quality management.
Design: A product can be significantly improved by design. For example, goods should be made with the right materials to ensure functionality and a longer shelf life.
Quality control: The level of quality is improved when waste and product defects are reduced during the QC process.
Quality management: Completing production processes that follow regulatory standards is at the core of quality management.
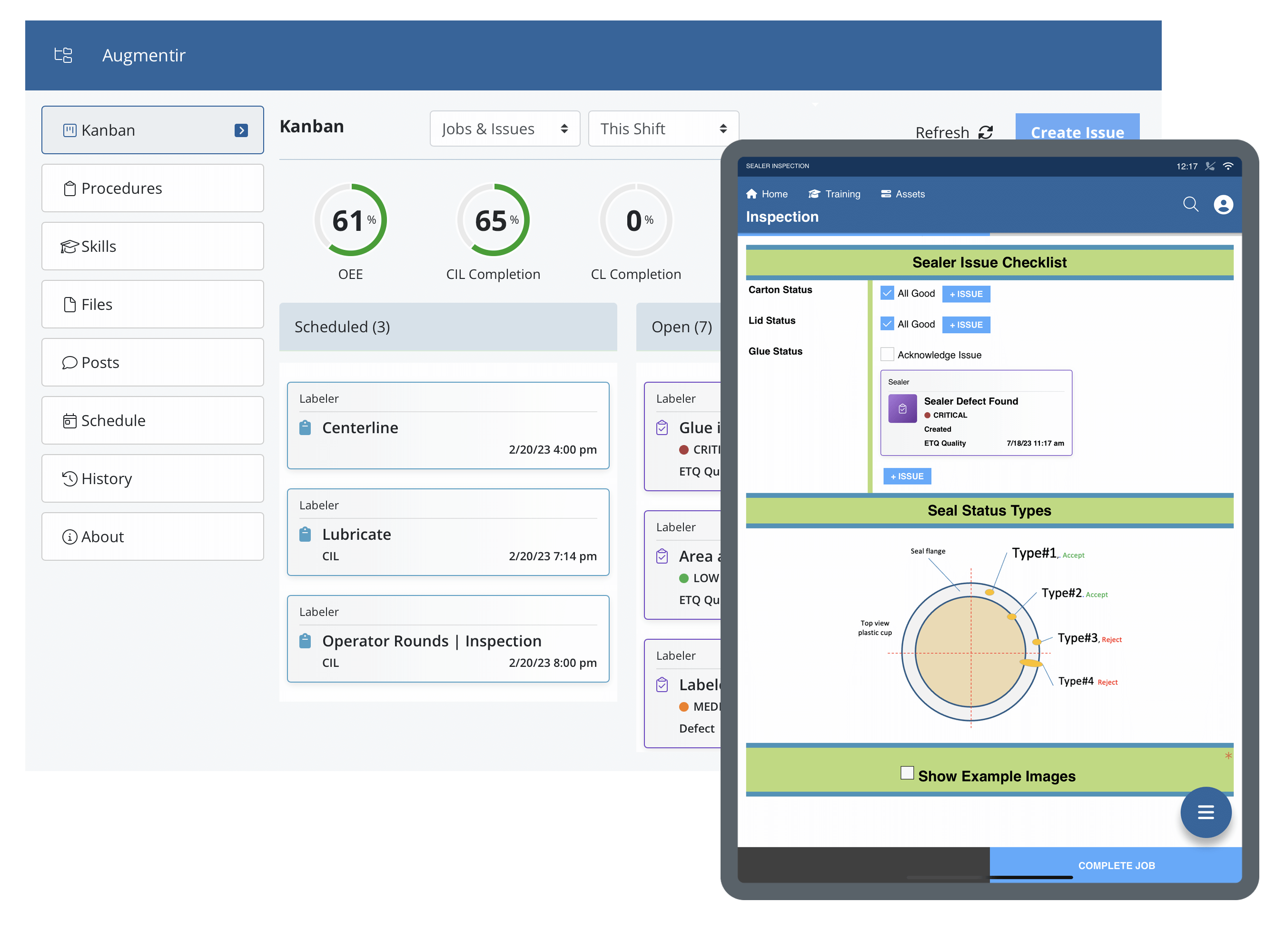
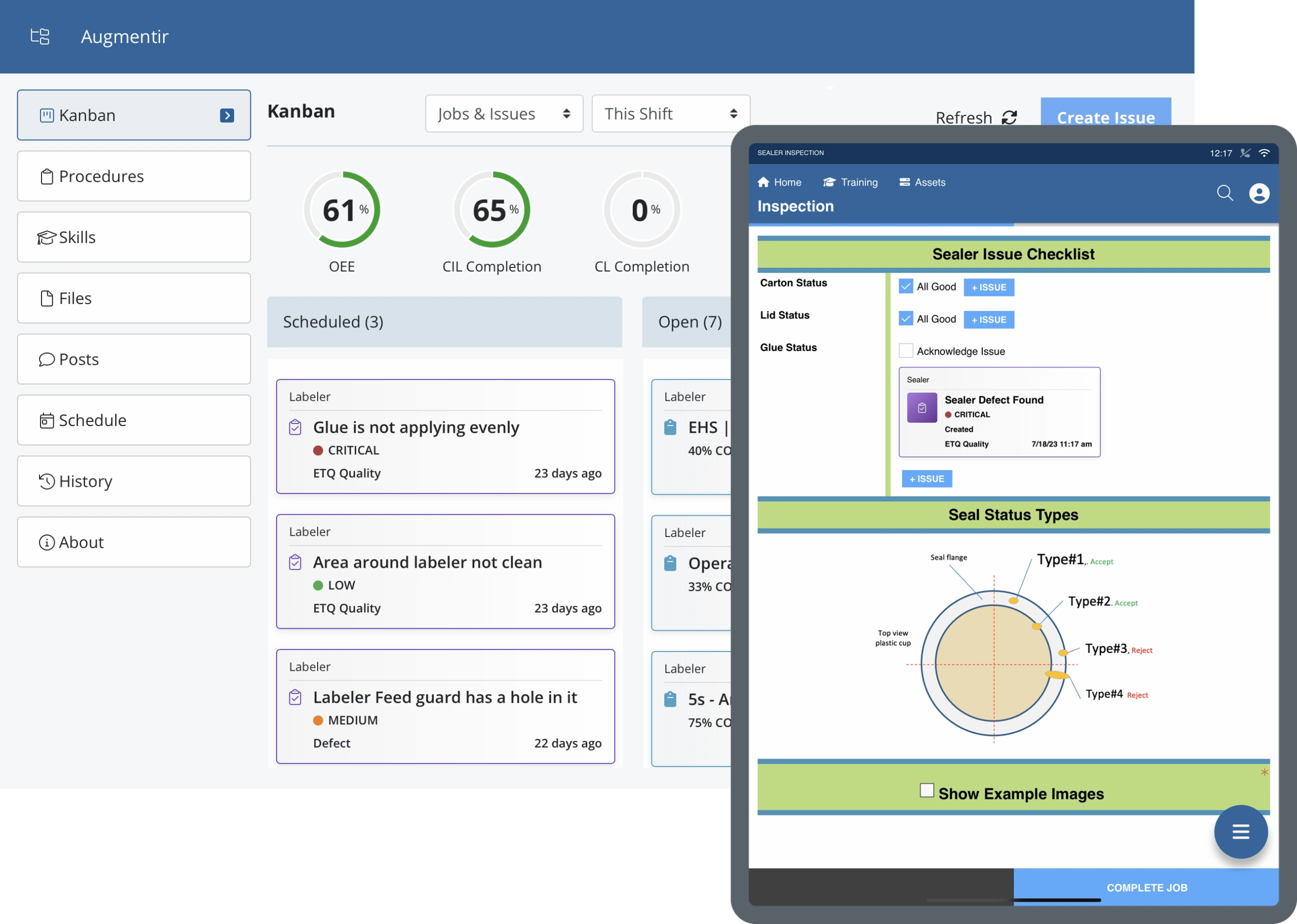
By digitizing quality control and quality assurance procedures, manufacturers can ensure a standardized approach towards inspections and quality data collection and improve overall compliance with quality standards.
Quality affects every facet of manufacturing
Production quality is more than just distributing products that people will trust and buy. Though that may be a key factor, quality affects every aspect of manufacturing, from workplace risk management to machine upkeep and inspection.
Quality affects many aspects of production. Examples include the following:
- Risk management ensures products are safe to use by customers and follow safety protocols. Smart, connected worker solutions are able to improve risk management through standardization and optimization of quality checks.
- Regulatory compliance is a key component of quality and can help prevent delays in production and fines. With digitized processes in place, manufacturers are able to ensure workers have access to the correct procedures and that tasks are performed in a standardized manner to avoid errors and promote improved compliance.
- Waste reduction is possible when material resources are conserved and used accordingly in production processes. AI-powered analytics in conjunction with smart, connected worker solutions allow for improved, streamlined processes that are able to reduce waste and improve yield through optimized production.
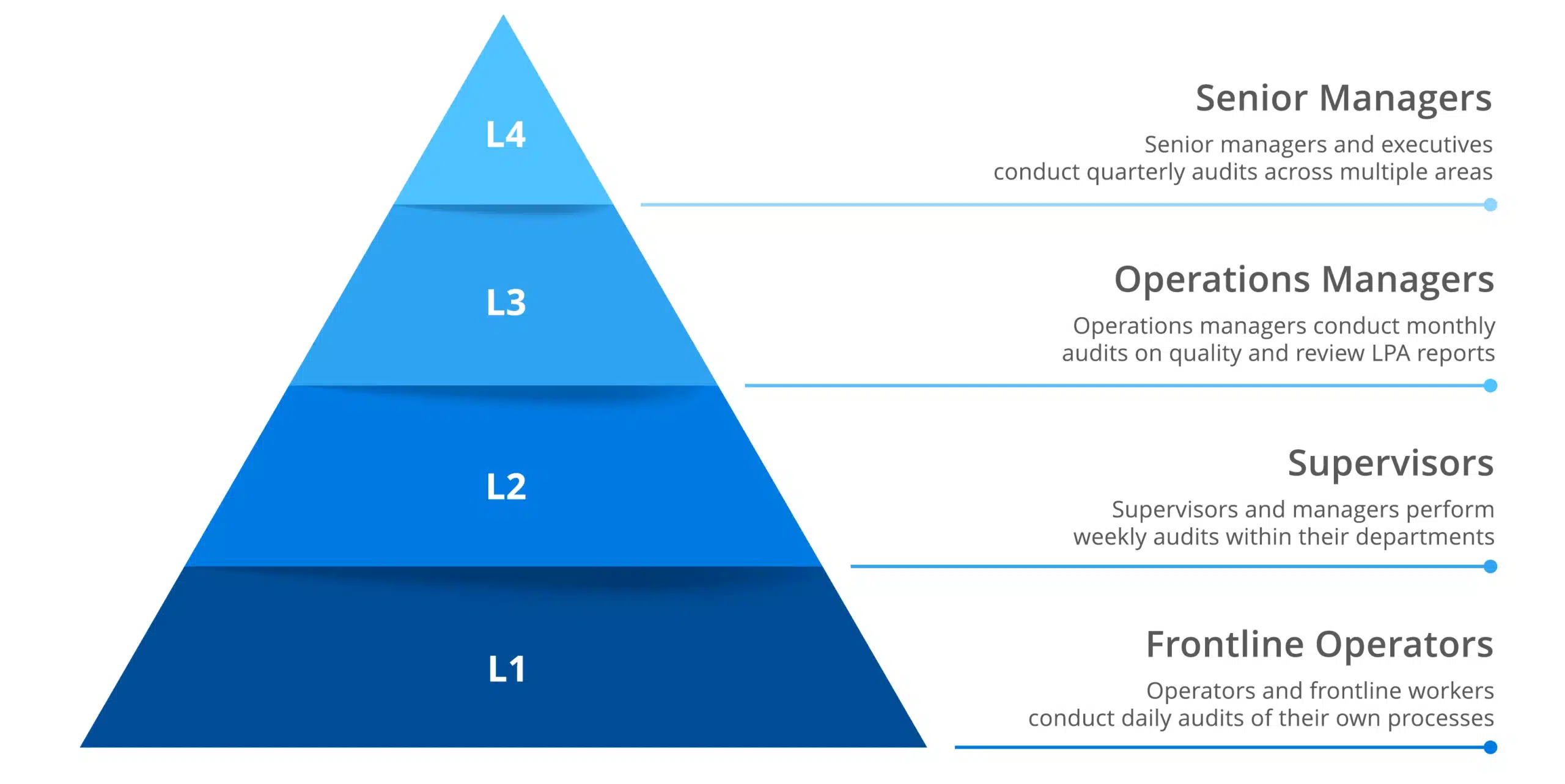
- Errors and defects are reduced when procedures are standardized using efficient QC processes to troubleshoot problems. With connected worker solutions and digitized quality control processes such as layered process audits, mistakes can be identified as they happen, protecting the production process.
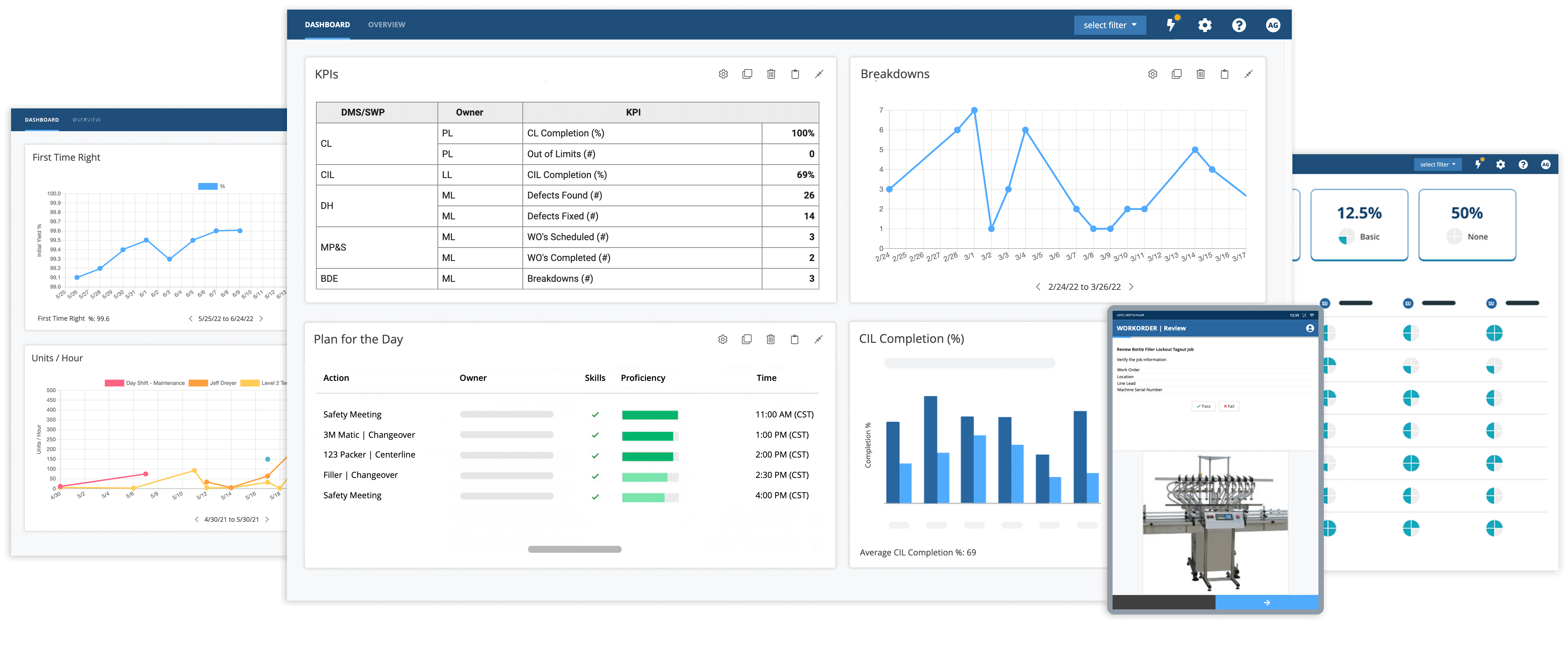
- Machine upkeep and inspection can be strengthened when industry best practices are implemented. Digitizing machine inspection standards and upkeep notifications and connecting frontline workers via smart, connected worker platforms gives operators the ability to practice preventative and autonomous maintenance and improves overall equipment effectiveness (OEE) and reduces unplanned downtime.
How to Improve Quality in Manufacturing
Quality improvement in manufacturing is vital to ensure a business is performing at its best. Here are some ways to boost quality with real-world examples:
Step 1: Practice lean manufacturing.
Lean manufacturing is the practice of reducing waste in production processes. Waste is defined as anything that does not bring value to the customer. This method requires an examination of your current practices to see which work and which leads to greater waste. The rise of digital technology is making it easier and more practical for manufacturers to connect and digitize their operations and drive further improvements and enhance lean manufacturing strategies.
Real-world application: An injection molding machine was found clogged with mold and was producing products with damaged seams. After resolving this issue by cleaning the machine, the company had less wasted plastic and fewer product malfunctions. With digitized notifications, real-time collaboration, and smart, connected worker solutions, situations like the above can be solved quickly and with reduced impact on production.
Step 2: Implement total productive maintenance.
Total Productive Maintenance (TPM) focuses on the idea that every employee should do their part to maximize equipment effectiveness. The objective is to create a culture where every worker adjusts and maintains machinery over the course of each shift. Through a combination of digital work instructions and real-time collaboration tools, manufacturers can better implement and improve TPM initiatives. This allows operators to independently complete maintenance tasks at peak performance and improve overall equipment effectiveness (OEE).
Real-world application: Both operators and maintenance staff can perform routine maintenance to check for errors or deficiencies. By implementing connected worker solutions organizations can improve the quality, transparency, and efficiency of maintenance and repair procedures and minimize machine downtime and reduce overall maintenance costs and impact.
Step 3: Embrace statistical process control.
This method involves detecting production issues by studying data anomalies to get rid of root causes before they ruin entire assembly lines. With connected frontline worker solutions that are integrated with enterprise quality management systems, organizations can improve statistical process control by optimizing data collection and inspection procedures through their frontline workforce. This essentially transforms frontline workers into quality sensors that further enhance and empower overall quality efforts.
Real-world application: Tracking the number of defective goods on each production line can help with identifying the root of any issue and taking corrective action. Smart, connected worker technology improves tracking ability, optimizes data collection, and identifies issues faster, reducing the risk of product recalls, and preserving consumer trust.
Digitizing Quality in Manufacturing with Augmentir
Companies are adopting innovative new technologies, processes, and methods to improve quality, productivity, and collaboration efforts across the industrial arena. Guaranteeing quality in manufacturing boils down to standardizing processes. Every procedure should contribute to product value and be carried out in a unified way. Implementing smart, connected solutions and coupling them with AI-powered analytics opens new paths for manufacturers to step forward and improve how they approach quality in the production process and beyond.
By digitizing analog paper practices, you enable better quality control and standardization of inspection procedures which, in turn, strengthens your overall manufacturing operations. Augmentir can help with the digitization and transformation process. We understand the need for effective quality control, and we have demonstrated success in helping manufacturers improve quality on the production floor.
Check out our quality use cases, and request a live demo today to learn for yourself why companies are choosing Augmentir to help standardize and digitize quality control procedures.